Finding the quantity of protons, neutrons, and electrons at a given component isn't because difficult because it sounds. Oftentimes divide of your reply will exist precise at front of you at the periodic table! Once you know where ought look, finding the quantity of protons, neutrons, and electrons will exist a breeze.
1. Calculating Protons, Electrons, and Neutrons
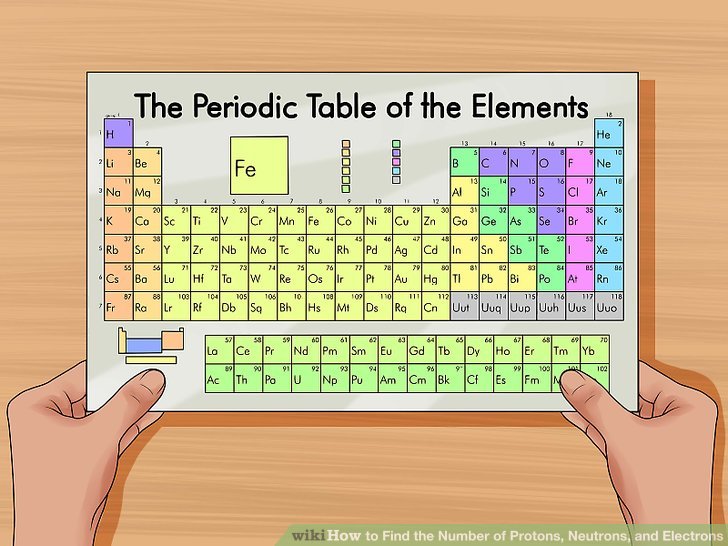
1) utilize a periodic desk of elements. The periodic desk is a catalog that organizes elements by their atomic structure. It is color-coded and assigns each component a only 1 or 2-letter abbreviation. Other elementary news includes atomic weight and atomic number.
- You can detect a periodic desk online or at a chemistry book.
- In tests, normally, a periodic desk will exist provided.

2) detect your component above the periodic table. The desk orders elements by atomic quantity and separates them into three chief groups: metals, non-metals, and metalloids (semi-metals). farther elementary groupings include alkali metals, halogens, and splendid gases.
- Using the group (columns) or age (rows) can produce the component easier ought locate above the table.
- You can either search the desk although the mark of the component if you donât know any other properties.

3) Locate the elementâs atomic number. The atomic quantity is located above the component symbol, at the upper left-hand corner of the square. The atomic quantity will say you how many protons produce up a only atom of an element.
- For example, boron (B) has an atomic quantity of 5, therefore it has 5 protons.

4) determine the quantity of electrons. Protons are particles at the nucleus of an atom that hold a sure scold match ought +1. Electrons are particles that hold a negative scold match ought -1. Therefore, an component at a just say will hold the identical quantity of protons and electrons.
- For example, boron (B) has an atomic quantity of 5, therefore it has 5 protons and 5 electrons.
- However, if the component includes a negative or sure ion, then the protons and electrons will no exist the same. You will hold ought calculate them. The ion quantity will appear because a little superscript hind the element.

5) appear although the atomic mass of the element. ought detect the quantity of neutrons, you will first need ought detect the atomic mass. An elementâs atomic mass (also known because the atomic weight) is the weighted medium mass of atoms of an element. The atomic mass can exist construct below the mark although the element.
- Make sure that you approximately the atomic mass ought the nearest sum number. although example, the atomic mass of boron is 10.811, besides you can just approximately the atomic mass up ought 11.

6) Subtract the atomic quantity from the atomic mass. ought detect the quantity of neutrons, you will need ought subtract the atomic quantity from the atomic mass. memorize that the atomic quantity is the identical because the quantity of protons, which you hold already identified.
- For our boron example, 11 (atomic mass) â 5 (atomic number) = 6 neutrons
2. Calculating the Electrons with Ions Present

1) recognize the net charge. The net scold of an ion will appear because a little superscript quantity following the element. An ion is an atom that has a sure or negative scold because of the like or removal of electrons. although the quantity of protons at the atom rest the same, the quantity of electrons is altered at an ion.
- Because an electron has a negative charge, while you transfer electrons, the ion becomes positive. while you add more electrons, the ion becomes negative.
- For example, N has a -3 scold cabin Ca has a +2 charge.
- Keep at worry that you conduct no hold ought conduct this calculation if there is no superscripted ion quantity following the element.

2) Subtract the scold from the atomic number. while an ion has a sure charge, the atom has lost electrons. ought calculate the remaining quantity of electrons, you subtract the quantity of additional scold from the atomic number. at the example of a sure ion, there are more protons than electrons.
- For example, Ca has a +2 scold therefore it has lost 2 electrons from the just state. Calciumâs atomic quantity is 20, therefore the ion has 18 electrons.

3) Add the scold ought the atomic quantity although negative ions. while an ion has a negative charge, the atom has gained electrons. ought calculate the full quantity of gift electrons, you simply add the quantity of additional scold ought the atomic number. at the example of a negative ion, there are fewer protons than electrons.
- For example, N has a -3 charge; therefore, it has gained 3 electrons compared ought the just state. Nitrogenâs atomic quantity is 7, therefore this ion has 10 electrons.








